主要信息
Target
DYH2
Host Species
Rabbit
Reactivity
Human, Mouse
Applications
IHC, IF
MW
487kD (Calculated)
Conjugate/Modification
Unmodified
货号: YT6443
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
IHC 1:50-200; IF 1:50-200
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
This antibody detects endogenous levels of DYH2 at Human/Mouse
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
理论分子量
487kD
修饰
Unmodified
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
Synthesized peptide derived from human DYH2 AA range: 329-379
展开内容
特异性:
This antibody detects endogenous levels of DYH2 at Human/Mouse
展开内容
基因名称:
DNAH2 DNAHC2 DNHD3 KIAA1503
展开内容
蛋白名称:
DYH2
展开内容
背景:
Dyneins are microtubule-associated motor protein complexes composed of several heavy, light, and intermediate chains. The axonemal dyneins, found in cilia and flagella, are components of the outer and inner dynein arms attached to the peripheral microtubule doublets. DNAH2 is an axonemal inner arm dynein heavy chain (Chapelin et al., 1997 [PubMed 9256245]).[supplied by OMIM, Mar 2008],
展开内容
功能:
Domain:Dynein heavy chains probably consist of an N-terminal stem (which binds cargo and interacts with other dynein components), and the head or motor domain. The motor contains six tandemly-linked AAA domains in the head, which form a ring. A stalk-like structure (formed by two of the coiled coil domains) protrudes between AAA 4 and AAA 5 and terminates in a microtubule-binding site. A seventh domain may also contribute to this ring; it is not clear whether the N-terminus or the C-terminus forms this extra domain. There are four well-conserved and two non-conserved ATPase sites, one per AAA domain. Probably only one of these (within AAA 1) actually hydrolyzes ATP, the others may serve a regulatory function.,Function:Force generating protein of respiratory cilia. Produces force towards the minus ends of microtubules. Dynein has ATPase activity; the force-producing power stroke is thought to occur on release of ADP. Involved in sperm motility; implicated in sperm flagellar assembly.,similarity:Belongs to the dynein heavy chain family.,similarity:Contains 5 LRR (leucine-rich) repeats.,similarity:Contains 5 TPR repeats.,subunit:Consists of at least two heavy chains and a number of intermediate and light chains.,tissue specificity:Expressed primarily in trachea and testis, 2 tissues containing axonemal structures. Also expressed in lung.,
展开内容
细胞定位:
Cytoplasm, cytoskeleton, cilium axoneme . Cytoplasm, cytoskeleton, flagellum axoneme .
展开内容
组织表达:
Expressed primarily in trachea and testis, 2 tissues containing axonemal structures. Also expressed in lung. Expressed in spermatozoa (at protein level) (PubMed:31178125).
展开内容
研究领域:
>>Amyotrophic lateral sclerosis ;
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
>>Huntington disease ;
>>Pathways of neurodegeneration - multiple diseases
展开内容
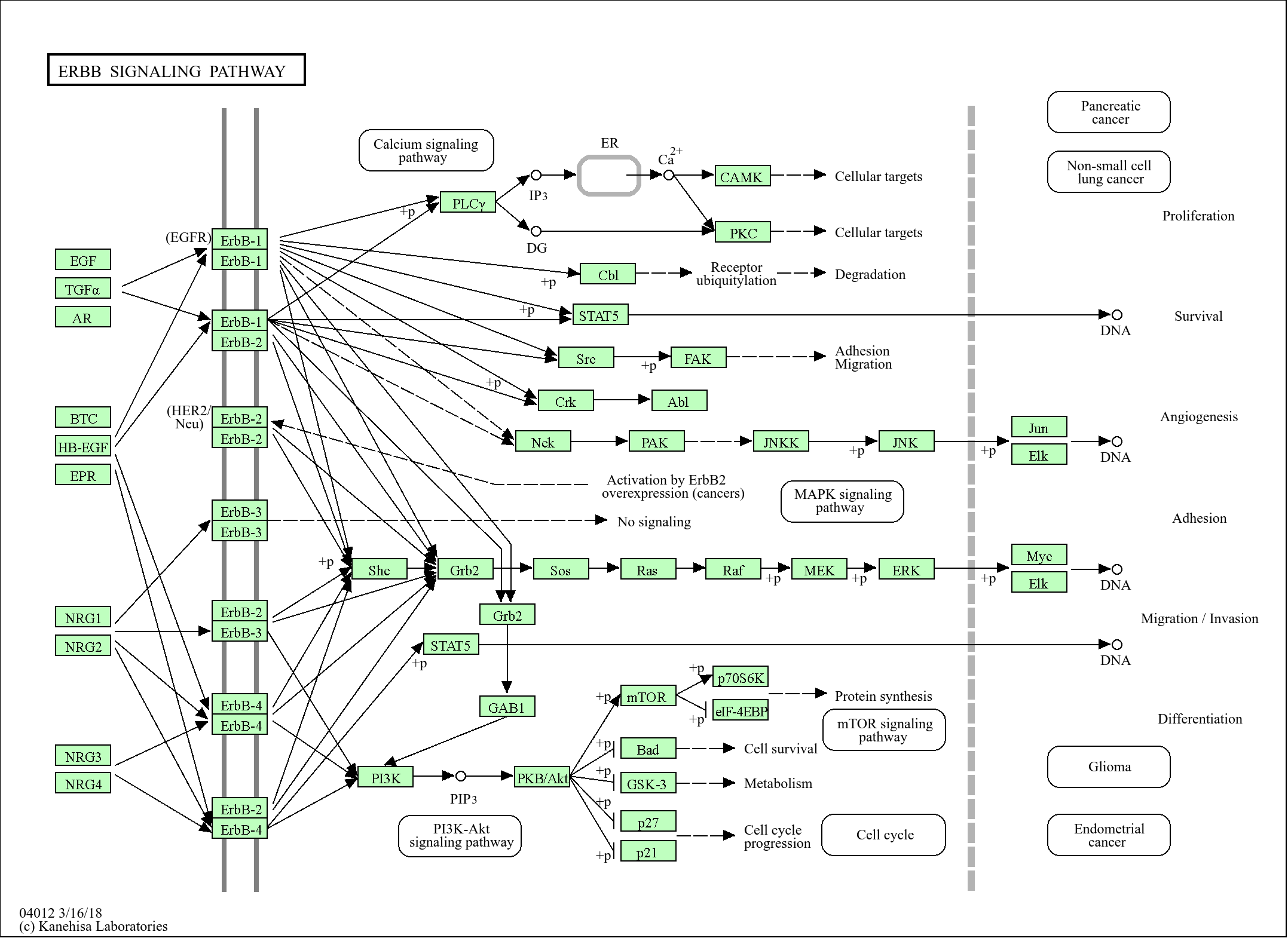
信号通路
文献引用({{totalcount}})
货号: YT6443
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}