主要信息
Target
PAH
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, ELISA
MW
51kD (Observed)
Conjugate/Modification
Unmodified
货号: YT3568
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:500-1:2000; IHC 1:100-1:300; ELISA 1:40000; IF 1:50-200
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
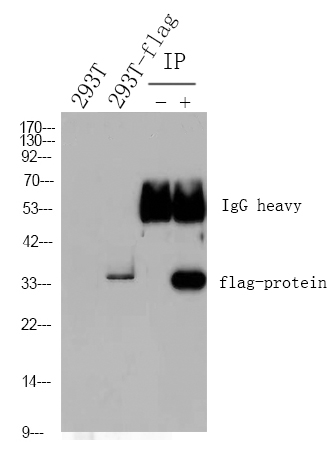
PAH Polyclonal Antibody detects endogenous levels of PAH protein.
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
实测条带
51kD
修饰
Unmodified
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
The antiserum was produced against synthesized peptide derived from human PAH. AA range:351-400
展开内容
特异性:
PAH Polyclonal Antibody detects endogenous levels of PAH protein.
展开内容
基因名称:
PAH
展开内容
蛋白名称:
Phenylalanine-4-hydroxylase
展开内容
别名:
PAH ;
Phenylalanine-4-hydroxylase ;
PAH ;
Phe-4-monooxygenase
Phenylalanine-4-hydroxylase ;
PAH ;
Phe-4-monooxygenase
展开内容
背景:
PAH encodes the enzyme phenylalanine hydroxylase that is the rate-limiting step in phenylalanine catabolism. Deficiency of this enzyme activity results in the autosomal recessive disorder phenylketonuria. [provided by RefSeq, Jul 2008],
展开内容
功能:
Catalytic activity:L-phenylalanine + tetrahydrobiopterin + O(2) = L-tyrosine + 4a-hydroxytetrahydrobiopterin.,cofactor:Fe(2+) ion.,Disease:Defects in PAH are the cause of hyperphenylalaninemia (HPA) [MIM:261600]. HPA is the mildest form of phenylalanine hydroxylase deficiency.,Disease:Defects in PAH are the cause of non-phenylketonuria hyperphenylalaninemia (Non-PKU HPA) [MIM:261600]. Non-PKU HPA is a mild form of phenylalanine hydroxylase deficiency characterized by phenylalanine levels persistently below 600 mumol, which allows normal intellectual and behavioral development without treatment. Non-PKU HPA is usually caused by the combined effect of a mild hyperphenylalaninemia mutation and a severe one.,Disease:Defects in PAH are the cause of phenylketonuria (PKU) [MIM:261600]. PKU is an autosomal recessive inborn error of phenylalanine metabolism, due to severe phenylalanine hydroxylase deficiency. It is characterized by blood concentrations of phenylalanine persistently above 1200 mumol (normal concentration 100 mumol) which usually causes mental retardation (unless low phenylalanine diet is introduced early in life). They tend to have light pigmentation, rashes similar to eczema, epilepsy, extreme hyperactivity, psychotic states and an unpleasant 'mousy' odor.,enzyme regulation:N-terminal region of PAH is thought to contain allosteric binding sites for phenylalanine and to constitute an "inhibitory" domain that regulates the activity of a catalytic domain in the C-terminal portion of the molecule.,online information:Phenylalanine hydroxylase entry,online information:Phenylalanine hydroxylase locus knowledgebase,pathway:Amino-acid degradation; L-phenylalanine degradation; acetoacetic acid and fumarate from L-phenylalanine: step 1/6.,polymorphism:The Glu-274 variant occurs on approximately 4% of African-American PAH alleles. The enzyme activity of the variant protein is indistinguishable from that of the wild-type form.,similarity:Belongs to the biopterin-dependent aromatic amino acid hydroxylase family.,similarity:Contains 1 ACT domain.,subunit:Homodimer.,
展开内容
细胞定位:
cytosol,extracellular exosome,
展开内容
组织表达:
Liver,
展开内容
研究领域:
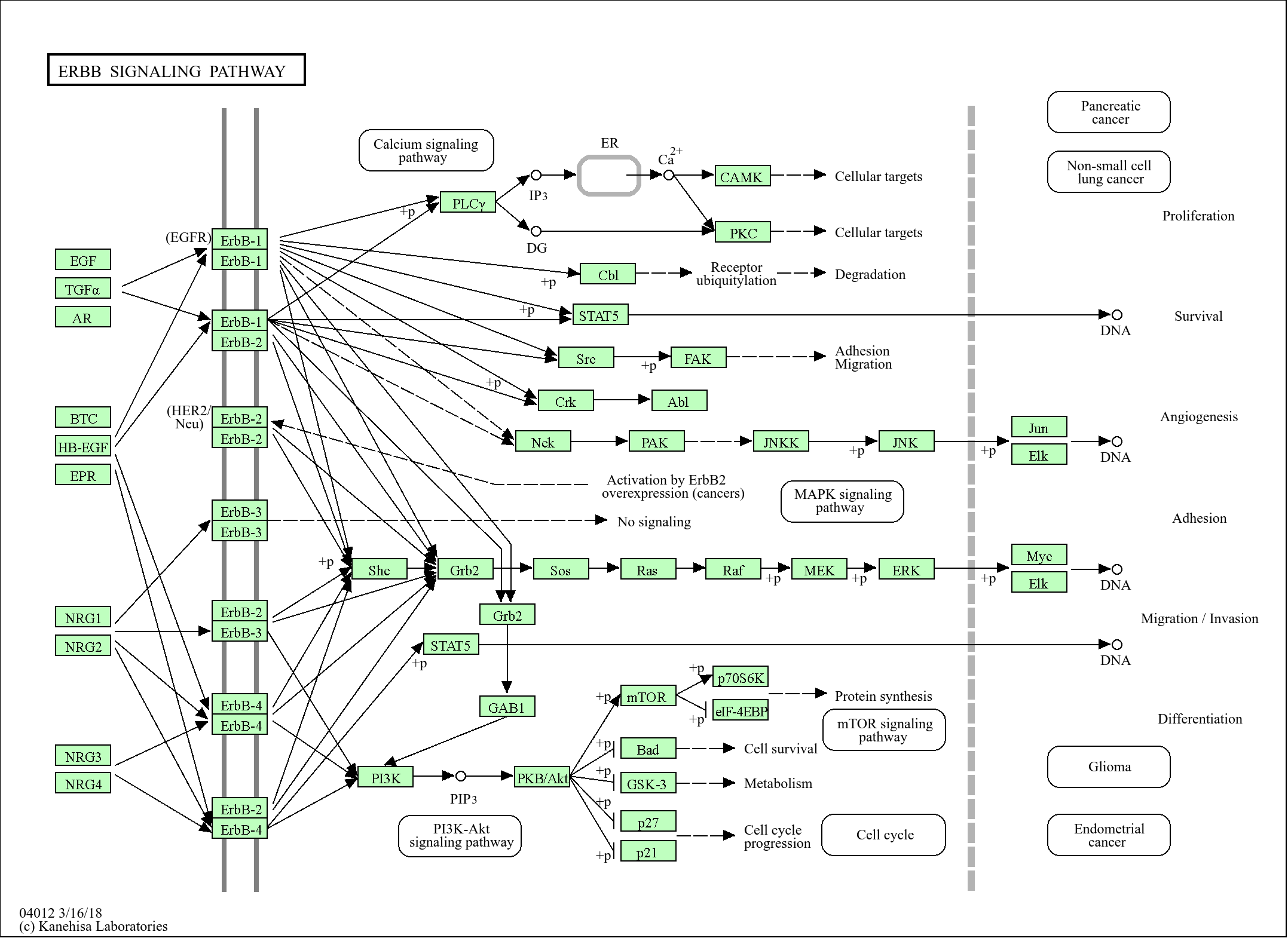
>>Phenylalanine metabolism ;
>>Phenylalanine, tyrosine and tryptophan biosynthesis ;
>>Folate biosynthesis ;
>>Metabolic pathways ;
>>Biosynthesis of amino acids
>>Phenylalanine, tyrosine and tryptophan biosynthesis ;
>>Folate biosynthesis ;
>>Metabolic pathways ;
>>Biosynthesis of amino acids
展开内容
文献引用({{totalcount}})
货号: YT3568
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}