主要信息
Target
HDAC8
Host Species
Rabbit
Reactivity
Human, Mouse, Rat, Monkey
Applications
WB, IHC, IF, ELISA
MW
42kD (Observed)
Conjugate/Modification
Unmodified
货号: YT2120
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:500-1:2000; IHC 1:100-1:300; ELISA 1:20000; IF 1:50-200
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
HDAC8 Polyclonal Antibody detects endogenous levels of HDAC8 protein.
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
实测条带
42kD
修饰
Unmodified
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
The antiserum was produced against synthesized peptide derived from human HDAC8. AA range:5-54
展开内容
特异性:
HDAC8 Polyclonal Antibody detects endogenous levels of HDAC8 protein.
展开内容
基因名称:
HDAC8
展开内容
蛋白名称:
Histone deacetylase 8
展开内容
别名:
HDAC8 ;
HDACL1 ;
CDA07 ;
Histone deacetylase 8 ;
HD8
HDACL1 ;
CDA07 ;
Histone deacetylase 8 ;
HD8
展开内容
背景:
Histones play a critical role in transcriptional regulation, cell cycle progression, and developmental events. Histone acetylation/deacetylation alters chromosome structure and affects transcription factor access to DNA. The protein encoded by this gene belongs to class I of the histone deacetylase family. It catalyzes the deacetylation of lysine residues in the histone N-terminal tails and represses transcription in large multiprotein complexes with transcriptional co-repressors. Multiple transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Oct 2009],
展开内容
功能:
Catalytic activity:Hydrolysis of an N(6)-acetyl-lysine residue of a histone to yield a deacetylated histone.,Caution:The sequence shown here is derived from an Ensembl automatic analysis pipeline and should be considered as preliminary data.,Function:Responsible for the deacetylation of lysine residues on the N-terminal part of the core histones (H2A, H2B, H3 and H4). Histone deacetylation gives a tag for epigenetic repression and plays an important role in transcriptional regulation, cell cycle progression and developmental events. Histone deacetylases act via the formation of large multiprotein complexes.,miscellaneous:Its activity is inhibited by trichostatin A (TSA) and butyrate, two well known histone deacetylase inhibitors.,similarity:Belongs to the histone deacetylase family. Type 1 subfamily.,subcellular location:Excluded from the nucleoli.,subunit:Interacts with PEPB2-MYH11, a fusion protein consisting of the 165 N-terminal residues of CBF-beta (PEPB2) with the tail region of MYH11 produced by the inversion Inv(16)(p13q22), a translocation associated with acute myeloid leukemia of M4EO subtype. The PEPB2-MYH1 fusion protein also interacts with RUNX1, a well known transcriptional regulator, suggesting that the interaction with HDAC8 may participate in the conversion of RUNX1 into a constitutive transcriptional repressor. Interacts with CBFA2T3.,tissue specificity:Weakly expressed in most tissues. Expressed at higher level in heart, brain, kidney and pancreas.,
展开内容
细胞定位:
Nucleus . Chromosome . Cytoplasm . Excluded from the nucleoli (PubMed:10748112). Found in the cytoplasm of cells showing smooth muscle differentiation (PubMed:15772115, PubMed:16538051). .
展开内容
研究领域:
>>Neutrophil extracellular trap formation ;
>>Alcoholism ;
>>Viral carcinogenesis
>>Alcoholism ;
>>Viral carcinogenesis
展开内容
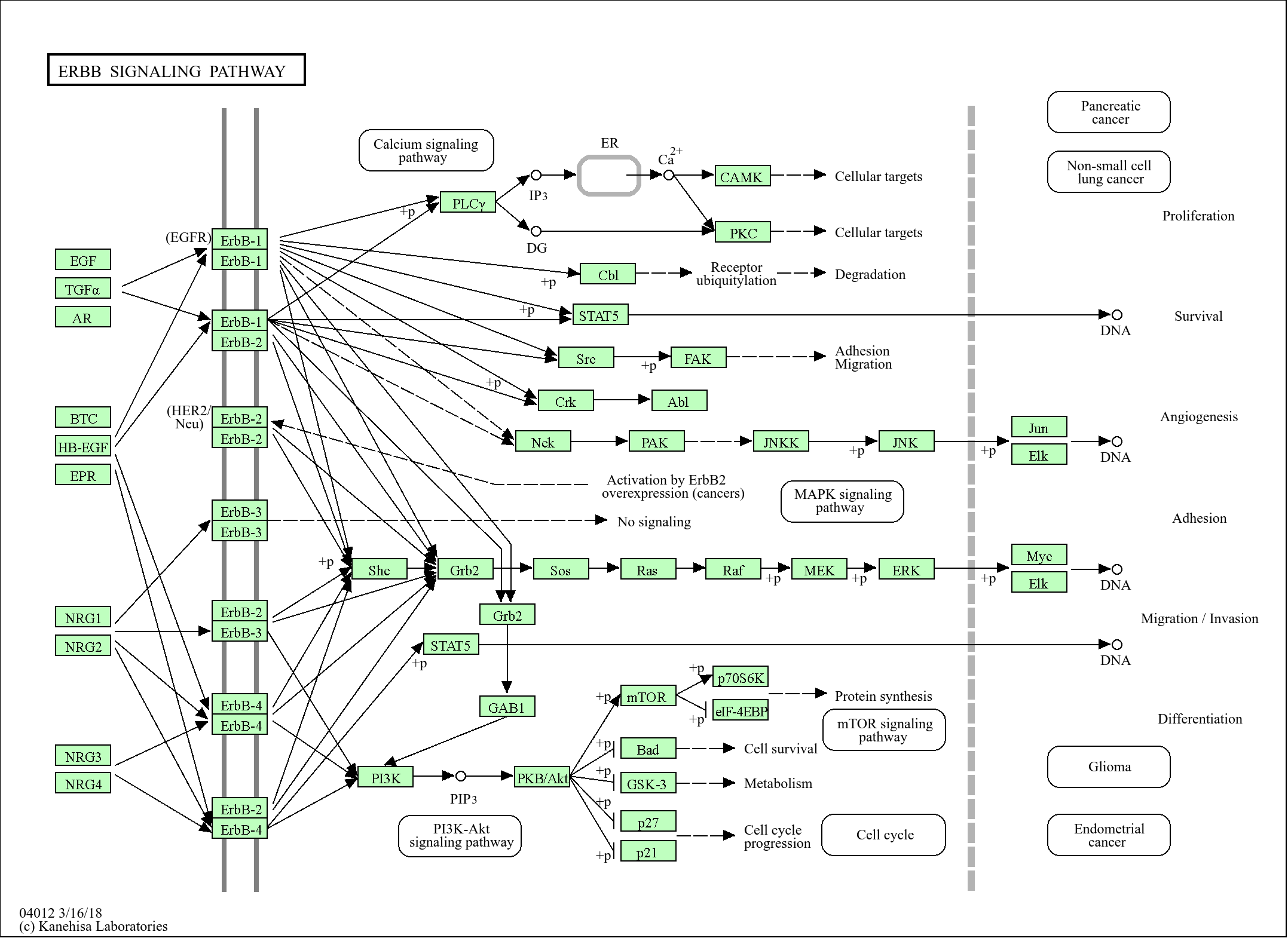
信号通路
文献引用({{totalcount}})
货号: YT2120
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}