主要信息
Target
eIF4G
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB
MW
180kD (Observed)
Conjugate/Modification
Phospho

货号: YP1328
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:1000-2000
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
This antibody detects endogenous levels of Human Mouse Rat eIF4G (phospho-Ser1108)
纯化工艺
The antibody was affinity-purified from rabbit serum by affinity-chromatography using specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
实测条带
180kD
修饰
Phospho
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
Synthesized phosho peptide around human eIF4G (Ser1108)
展开内容
特异性:
This antibody detects endogenous levels of Human Mouse Rat eIF4G (phospho-Ser1108)
展开内容
基因名称:
EIF4G1 EIF4F EIF4G EIF4GI
展开内容
蛋白名称:
eIF4G (Ser1108)
展开内容
别名:
Eukaryotic translation initiation factor 4 gamma 1 ;
eIF-4-gamma 1 ;
eIF-4G 1 ;
eIF-4G1 ;
p220 ;
eIF-4-gamma 1 ;
eIF-4G 1 ;
eIF-4G1 ;
p220 ;
展开内容
背景:
The protein encoded by this gene is a component of the multi-subunit protein complex EIF4F. This complex facilitates the recruitment of mRNA to the ribosome, which is a rate-limiting step during the initiation phase of protein synthesis. The recognition of the mRNA cap and the ATP-dependent unwinding of 5'-terminal secondary structure is catalyzed by factors in this complex. The subunit encoded by this gene is a large scaffolding protein that contains binding sites for other members of the EIF4F complex. A domain at its N-terminus can also interact with the poly(A)-binding protein, which may mediate the circularization of mRNA during translation. Alternative splicing results in multiple transcript variants, some of which are derived from alternative promoter usage. [provided by RefSeq, Aug 2010],
展开内容
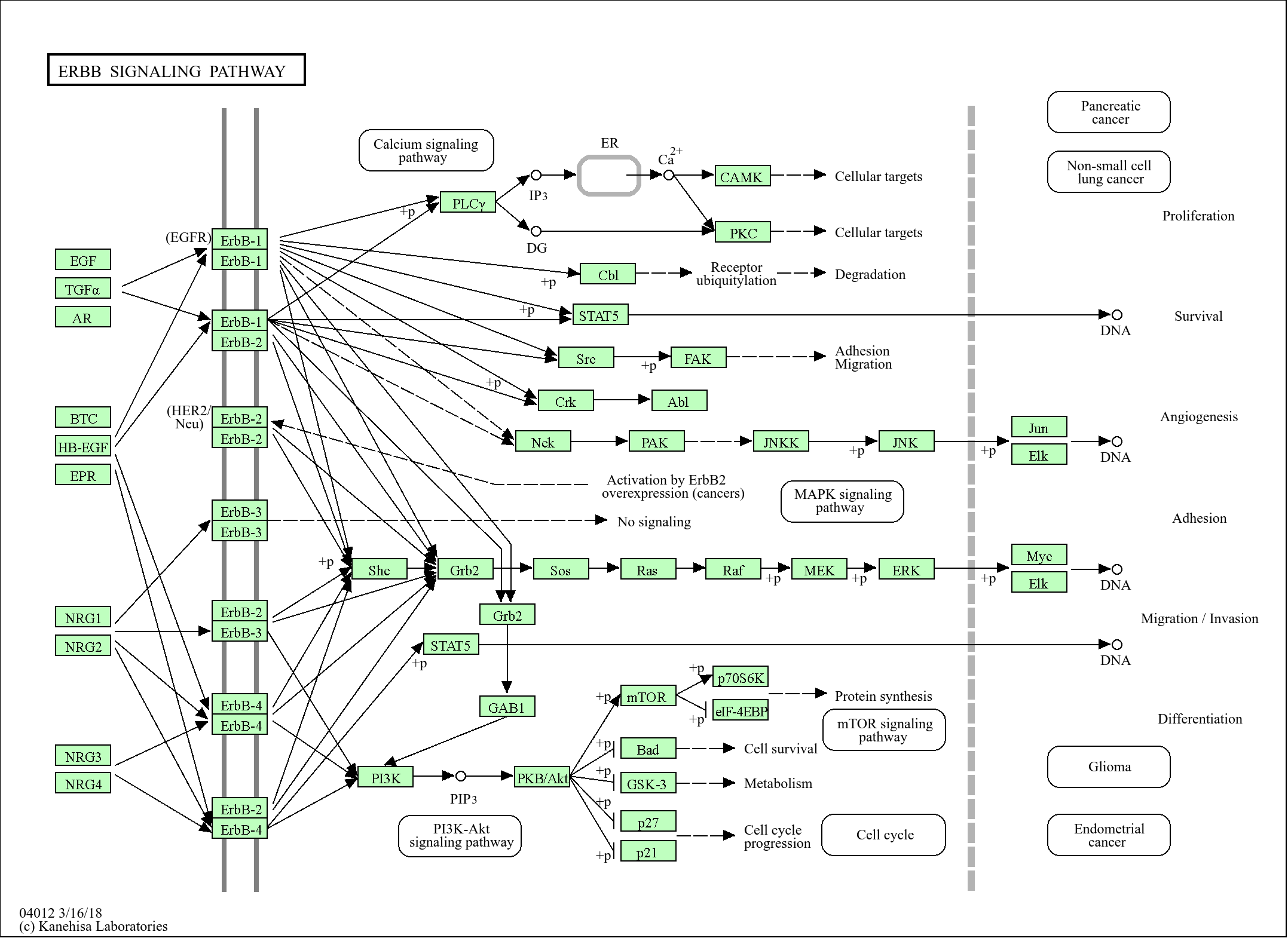
功能:
Function:Component of the protein complex eIF4F, which is involved in the recognition of the mRNA cap, ATP-dependent unwinding of 5'-terminal secondary structure and recruitment of mRNA to the ribosome.,PTM:Following infection by certain enteroviruses, rhinoviruses and aphthoviruses, EIF4G1 is cleaved by the viral protease 2A, or the leader protease in the case of aphthoviruses. This shuts down the capped cellular mRNA transcription.,PTM:Phosphorylated at multiple sites in vivo.,sequence Caution:Aberrant splicing.,similarity:Belongs to the eIF4G family.,similarity:Contains 1 MI domain.,similarity:Contains 1 MIF4G domain.,similarity:Contains 1 W2 domain.,subunit:eIF4F is a multi-subunit complex, the composition of which varies with external and internal environmental conditions. It is composed of at least EIF4A, EIF4E and EIF4G1/EIF4G3. Interacts with eIF3, mutually exclusive with EIF4A1 or EIFA2, EIF4E and through its N-terminus with PAPBC1. Interacts through its C-terminus with the serine/threonine kinases MKNK1, and with MKNK2. Appears to act as a scaffold protein, holding these enzymes in place to phosphorylate EIF4E. Non-phosphorylated EIF4EBP1 competes with EIF4G1/EIF4G3 to interact with EIF4E; insulin stimulated MAP-kinase (MAPK1 and MAPK3) phosphorylation of EIF4EBP1 causes dissociation of the complex allowing EIF4G1/EIF4G3 to bind and consequent initiation of translation. EIF4G1/EIF4G3 interacts with PABPC1 to bring about circularization of the mRNA. Rapamycin can attenuate insulin stimulation mediated by FKBPs. Interacts with EIF4E3. Interacts with MIF4GD. Interacts with rotavirus A NSP3; in this interaction, NSP3 takes the place of PABPC1 thereby inducing shutoff of host protein synthesis.,
展开内容
细胞定位:
Cytoplasm, Stress granule .
展开内容
组织表达:
Brain,Endometrial tumor,Epithelium,Pancreas,Placent
展开内容
研究领域:
>>Viral myocarditis
展开内容
文献引用({{totalcount}})
货号: YP1328
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}