主要信息
Target
Crystallin-αB
Host Species
Rabbit
Reactivity
Human, Mouse, Rat, Monkey
Applications
WB, IHC, IF, ELISA
MW
24kD (Observed)
Conjugate/Modification
Phospho
货号: YP0736
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:500-1:2000; IHC 1:100-1:300; ELISA 1:10000; IF 1:50-200
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
Phospho-Crystallin-αB (S45) Polyclonal Antibody detects endogenous levels of Crystallin-αB protein only when phosphorylated at S45.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):SLsPF
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
实测条带
24kD
修饰
Phospho
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
The antiserum was produced against synthesized peptide derived from human CRYAB around the phosphorylation site of Ser45. AA range:21-70
展开内容
特异性:
Phospho-Crystallin-αB (S45) Polyclonal Antibody detects endogenous levels of Crystallin-αB protein only when phosphorylated at S45.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):SLsPF
展开内容
基因名称:
CRYAB
展开内容
蛋白名称:
Alpha-crystallin B chain
展开内容
别名:
CRYAB ;
CRYA2 ;
Alpha-crystallin B chain ;
Alpha ;
B ;
-crystallin ;
Heat shock protein beta-5 ;
HspB5 ;
Renal carcinoma antigen NY-REN-27 ;
Rosenthal fiber component
CRYA2 ;
Alpha-crystallin B chain ;
Alpha ;
B ;
-crystallin ;
Heat shock protein beta-5 ;
HspB5 ;
Renal carcinoma antigen NY-REN-27 ;
Rosenthal fiber component
展开内容
背景:
Mammalian lens crystallins are divided into alpha, beta, and gamma families. Alpha crystallins are composed of two gene products: alpha-A and alpha-B, for acidic and basic, respectively. Alpha crystallins can be induced by heat shock and are members of the small heat shock protein (HSP20) family. They act as molecular chaperones although they do not renature proteins and release them in the fashion of a true chaperone; instead they hold them in large soluble aggregates. Post-translational modifications decrease the ability to chaperone. These heterogeneous aggregates consist of 30-40 subunits; the alpha-A and alpha-B subunits have a 3:1 ratio, respectively. Two additional functions of alpha crystallins are an autokinase activity and participation in the intracellular architecture. The encoded protein has been identified as a moonlighting protein based on its ability to perform mechanistically distin
展开内容
功能:
Disease:Crystallins do not turn over as the lens ages, providing ample opportunity for post-translational modifications or oxidations. These modifications may change crystallin solubility properties and favor senile cataract.,Disease:Defects in CRYAB are the cause of alpha-B crystallinopathy [MIM:608810]. Alpha-B crystallinopathy is a an autosomal dominant form of desmin-related myopathy (DRM) that results in weakness of the proximal and distal limb muscle (including neck, velopharynx, and trunk muscles), signs of cardiomyopathy and cataract. Patients with progressive myopathy characterized by myofibrillar degeneration that commences at the Z-disk, have been described. Mutations truncate the essential C-terminal domain of the protein required for the chaperone function.,Disease:Seen as Rosenthal fiber protein in the brain tissue of patients with Alexander disease.,Function:May contribute to the transparency and refractive index of the lens.,mass spectrometry: PubMed:10930324,mass spectrometry: PubMed:8175657,mass spectrometry:With 1 phosphate group PubMed:10930324,mass spectrometry:With 1 phosphate group PubMed:8175657,mass spectrometry:With 2 phosphate groups PubMed:8175657,similarity:Belongs to the small heat shock protein (HSP20) family.,subunit:Aggregates with homologous proteins, including CRYAA and the small heat shock protein HSPB1, to form large heteromeric complexes. Interacts with HSPBAP1 and TTN/titin.,tissue specificity:Lens as well as other tissues.,
展开内容
细胞定位:
Cytoplasm . Nucleus . Secreted . Lysosome . Translocates to the nucleus during heat shock and resides in sub-nuclear structures known as SC35 speckles or nuclear splicing speckles (PubMed:19464326). Localizes at the Z-bands and the intercalated disk in cardiomyocytes (PubMed:28493373). Can be secreted; the secretion is dependent on protein unfolding and facilitated by the cargo receptor TMED10; it results in protein translocation from the cytoplasm into the ERGIC (endoplasmic reticulum-Golgi intermediate compartment) followed by vesicle entry and secretion (PubMed:32272059). .
展开内容
组织表达:
Lens as well as other tissues (PubMed:838078, PubMed:2387586). Expressed in myocardial tissue (PubMed:28493373).
展开内容
研究领域:
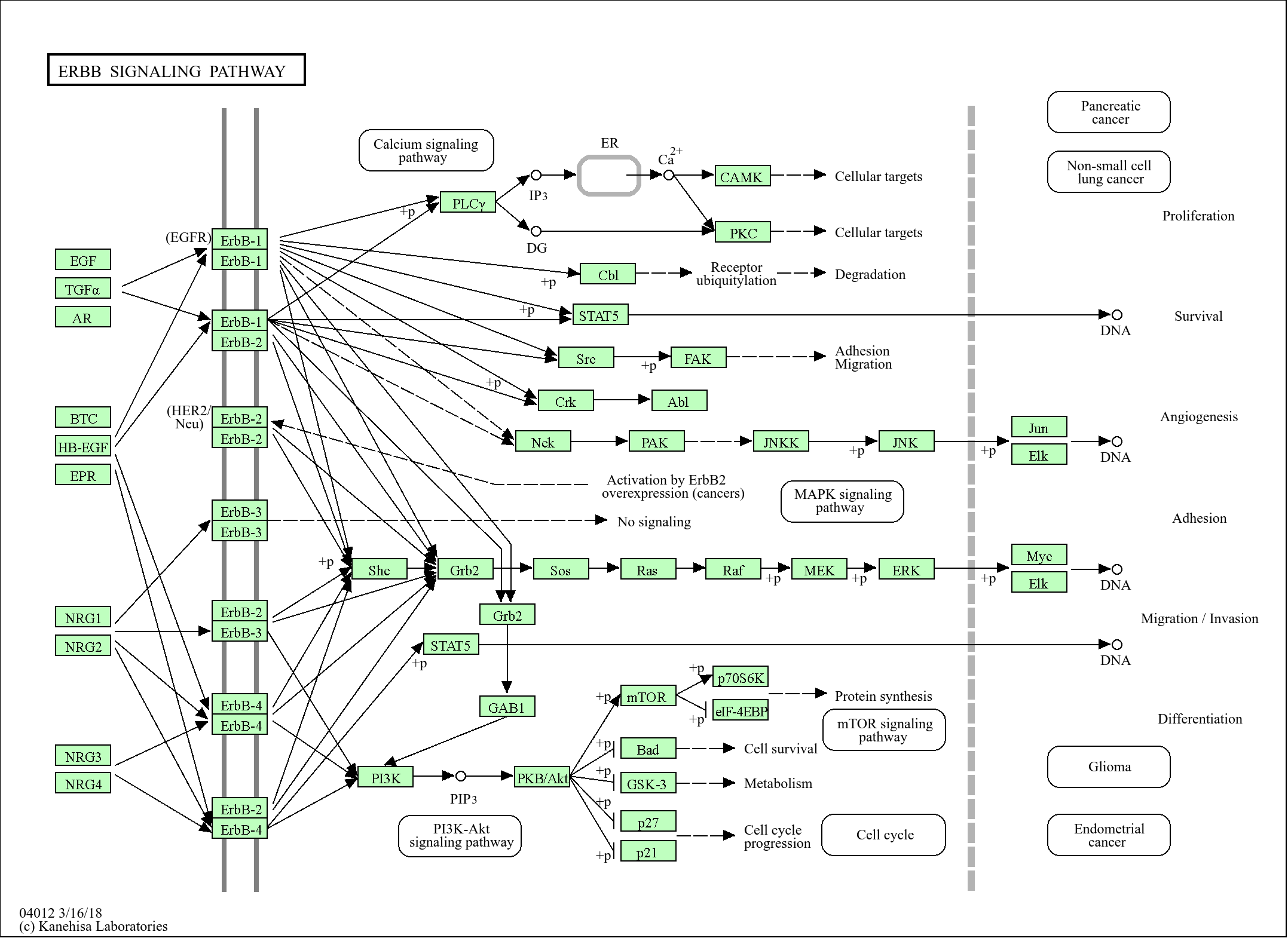
>>Protein processing in endoplasmic reticulum ;
>>Longevity regulating pathway - multiple species
>>Longevity regulating pathway - multiple species
展开内容
信号通路
文献引用({{totalcount}})
货号: YP0736
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}