主要信息
Target
Bcl-x
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, ELISA
MW
26kD (Calculated)
Conjugate/Modification
Phospho
货号: YP0034
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:500-1:2000; IHC 1:100-1:300; ELISA 1:10000; IF 1:50-200
Note:For IHC,wesuggest antigen retrieval with TE buffer pH 9.0 (Cat#YS0004)
Note:For IHC,wesuggest antigen retrieval with TE buffer pH 9.0 (Cat#YS0004)
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
Phospho-Bcl-x (S62) Polyclonal Antibody detects endogenous levels of Bcl-x protein only when phosphorylated at S62.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):ADsPA
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
理论分子量
26kD
修饰
Phospho
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
The antiserum was produced against synthesized peptide derived from human BCL-XL around the phosphorylation site of Ser62. AA range:28-77
展开内容
特异性:
Phospho-Bcl-x (S62) Polyclonal Antibody detects endogenous levels of Bcl-x protein only when phosphorylated at S62.The name of modified sites may be influenced by many factors, such as species (the modified site was not originally found in human samples) and the change of protein sequence (the previous protein sequence is incomplete, and the protein sequence may be prolonged with the development of protein sequencing technology). When naming, we will use the "numbers" in historical reference to keep the sites consistent with the reports. The antibody binds to the following modification sequence (lowercase letters are modification sites):ADsPA
展开内容
基因名称:
BCL2L1
展开内容
蛋白名称:
Bcl-2-like protein 1
展开内容
别名:
BCL2L1 ;
BCL2L ;
BCLX ;
Bcl-2-like protein 1 ;
Bcl2-L-1 ;
Apoptosis regulator Bcl-X
BCL2L ;
BCLX ;
Bcl-2-like protein 1 ;
Bcl2-L-1 ;
Apoptosis regulator Bcl-X
展开内容
背景:
The protein encoded by this gene belongs to the BCL-2 protein family. BCL-2 family members form hetero- or homodimers and act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. The proteins encoded by this gene are located at the outer mitochondrial membrane, and have been shown to regulate outer mitochondrial membrane channel (VDAC) opening. VDAC regulates mitochondrial membrane potential, and thus controls the production of reactive oxygen species and release of cytochrome C by mitochondria, both of which are the potent inducers of cell apoptosis. Alternative splicing results in multiple transcript variants encoding two different isoforms. The longer isoform acts as an apoptotic inhibitor and the shorter isoform acts as an apoptotic activator. [provided by RefSeq, Dec 2015],
展开内容
功能:
Domain:The BH4 motif is required for anti-apoptotic activity. The BH1 and BH2 motifs are required for both heterodimerization with other Bcl-2 family members and for repression of cell death.,Function:Potent inhibitor of cell death. Isoform Bcl-X(L) anti-apoptotic activity is inhibited by association with SIVA isoform 1. Inhibits activation of caspases (By similarity). Appears to regulate cell death by blocking the voltage-dependent anion channnel (VDAC) by binding to it and preventing the release of the caspase activator, cytochrome c, from the mitochondrial membrane. The Bcl-X(S) isoform promotes apoptosis.,PTM:Proteolytically cleaved by caspases during apoptosis. The cleaved protein, lacking the BH4 motif, has pro-apoptotic activity.,similarity:Belongs to the Bcl-2 family.,subcellular location:Mitochondrial membranes and perinuclear envelope.,subunit:Bcl-X(L) forms homodimers, and heterodimers with BAX, BAK and BCL2. Heterodimerization with BAX does not seem to be required for anti-apoptotic activity. Also interacts with BAD and BBC3. Isoform Bcl-X(L) binds to Siva isoform 1. Interacts with BCL2L11 (By similarity). Interacts with BECN1 and PGAM5. Isoform Bcl-X(L) interacts with BAX isoform Sigma.,tissue specificity:Bcl-X(S) is expressed at high levels in cells that undergo a high rate of turnover, such as developing lymphocytes. In contrast, Bcl-X(L) is found in tissues containing long-lived postmitotic cells, such as adult brain.,
展开内容
细胞定位:
[Isoform Bcl-X(L)]: Mitochondrion inner membrane . Mitochondrion outer membrane . Mitochondrion matrix . Cytoplasmic vesicle, secretory vesicle, synaptic vesicle membrane . Cytoplasm, cytosol . Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Nucleus membrane ; Single-pass membrane protein ; Cytoplasmic side . After neuronal stimulation, translocates from cytosol to synaptic vesicle and mitochondrion membrane in a calmodulin-dependent manner (By similarity). Localizes to the centrosome when phosphorylated at Ser-49. .
展开内容
组织表达:
Bcl-X(S) is expressed at high levels in cells that undergo a high rate of turnover, such as developing lymphocytes. In contrast, Bcl-X(L) is found in tissues containing long-lived postmitotic cells, such as adult brain.
展开内容
研究领域:
>>EGFR tyrosine kinase inhibitor resistance ;
>>Platinum drug resistance ;
>>Ras signaling pathway ;
>>NF-kappa B signaling pathway ;
>>p53 signaling pathway ;
>>Mitophagy - animal ;
>>Autophagy - animal ;
>>PI3K-Akt signaling pathway ;
>>Apoptosis ;
>>Apoptosis - multiple species ;
>>NOD-like receptor signaling pathway ;
>>JAK-STAT signaling pathway ;
>>Parkinson disease ;
>>Amyotrophic lateral sclerosis ;
>>Pathways of neurodegeneration - multiple diseases ;
>>Shigellosis ;
>>Toxoplasmosis ;
>>Measles ;
>>Human T-cell leukemia virus 1 infection ;
>>Herpes simplex virus 1 infection ;
>>Human immunodeficiency virus 1 infection ;
>>Pathways in cancer ;
>>Transcriptional misregulation in cancer ;
>>Pancreatic cancer ;
>>Chronic myeloid leukemia ;
>>Small cell lung cancer ;
>>Hepatocellular carcinoma ;
>>Lipid and atherosclerosis
>>Platinum drug resistance ;
>>Ras signaling pathway ;
>>NF-kappa B signaling pathway ;
>>p53 signaling pathway ;
>>Mitophagy - animal ;
>>Autophagy - animal ;
>>PI3K-Akt signaling pathway ;
>>Apoptosis ;
>>Apoptosis - multiple species ;
>>NOD-like receptor signaling pathway ;
>>JAK-STAT signaling pathway ;
>>Parkinson disease ;
>>Amyotrophic lateral sclerosis ;
>>Pathways of neurodegeneration - multiple diseases ;
>>Shigellosis ;
>>Toxoplasmosis ;
>>Measles ;
>>Human T-cell leukemia virus 1 infection ;
>>Herpes simplex virus 1 infection ;
>>Human immunodeficiency virus 1 infection ;
>>Pathways in cancer ;
>>Transcriptional misregulation in cancer ;
>>Pancreatic cancer ;
>>Chronic myeloid leukemia ;
>>Small cell lung cancer ;
>>Hepatocellular carcinoma ;
>>Lipid and atherosclerosis
展开内容
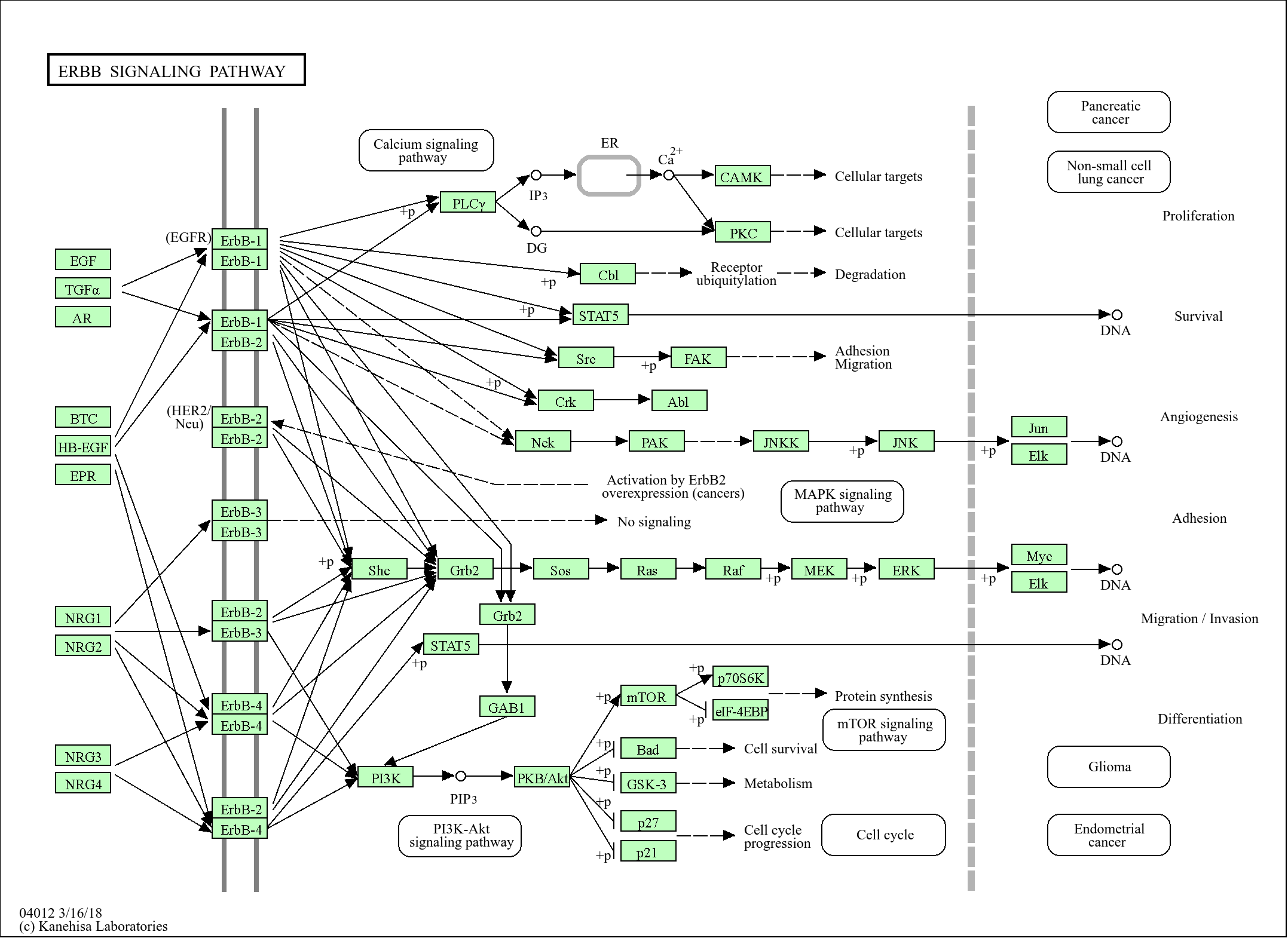
信号通路
Cellular Processes >> Transport and catabolism >> Autophagy - animal
Cellular Processes >> Transport and catabolism >> Mitophagy - animal
Cellular Processes >> Cell growth and death >> Apoptosis
Cellular Processes >> Cell growth and death >> Apoptosis - multiple species
Cellular Processes >> Cell growth and death >> p53 signaling pathway
Organismal Systems >> Immune system >> NOD-like receptor signaling pathway
Human Diseases >> Cancer: overview >> Pathways in cancer
Human Diseases >> Cancer: overview >> Transcriptional misregulation in cancer
Human Diseases >> Cancer: specific types >> Pancreatic cancer
Human Diseases >> Cancer: specific types >> Hepatocellular carcinoma
Human Diseases >> Cancer: specific types >> Chronic myeloid leukemia
Human Diseases >> Cancer: specific types >> Small cell lung cancer
Human Diseases >> Neurodegenerative disease >> Parkinson disease
Human Diseases >> Neurodegenerative disease >> Amyotrophic lateral sclerosis
Human Diseases >> Neurodegenerative disease >> Pathways of neurodegeneration - multiple diseases
Environmental Information Processing >> Signal transduction >> Ras signaling pathway
Environmental Information Processing >> Signal transduction >> JAK-STAT signaling pathway
Environmental Information Processing >> Signal transduction >> NF-kappa B signaling pathway
Environmental Information Processing >> Signal transduction >> PI3K-Akt signaling pathway
文献引用({{totalcount}})
货号: YP0034
规格
价格
货期
数量
200μL
¥4,680.00
现货
0
100μL
¥2,800.00
现货
0
50μL
¥1,500.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}